Chemical Industry

Wet chemical scrubber system
Gases generated from various chemical processes often contain pollutants that must be removed prior to emission into the environment. Examples of these pollutants include HCl, H2S, and SO2. These are often referred to as "acid gases".
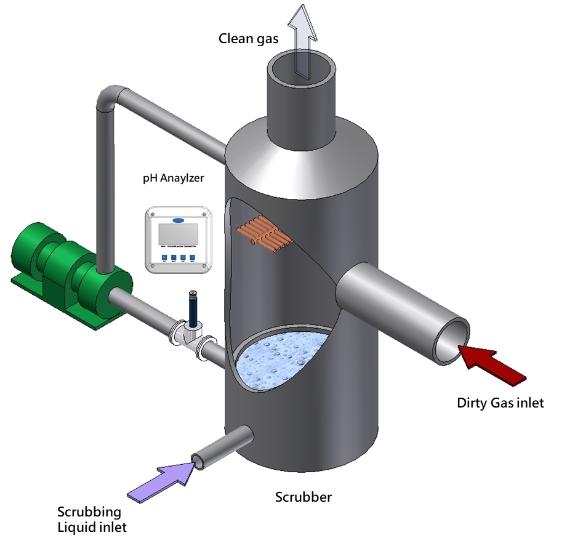
The gases pass through the scrubber where they are exposed to reagent chemicals that neutralize the pollutants. The reagents are sprayed in through carefully aligned nozzles within the scrubber tower. The spray creates a fine mist that mixes with the gas as it flows upwards through the tower. Excess liquids accumulate in the bottom of the scrubber where they are recirculated back for re-use.
pH measurement is used to determine the strength of the chemical reagents used in the scrubber. As the pH value trends towards acidic, fresh reagent will be added to the mix to increase the ability to neutralize the gas. Depending on the chemical reaction between the acid gas and the reagent, a solid precipitation may occur in the bottom of the scrubber. Proper pH control is also used to prevent these solids from possibly plugging the nozzles and reducing scubber efficiency.
Product Recommendation
- 2-wire pH/ORP analyzer
transmitter model: 96PH
sensor model: B65 (inline style)
B75 (insertion style) - 4-wire pH/ORP analyzer
transmitter model: 98PH
sensor model: B65 (inline style)
B75 (insertion style)
Product Recommendation
Chlor-Alkali process ,
Brine treatment &
Dechlorination
Brine treatment is the process of purification the brine for reuse and producing a concentrated brine. In chlor-alkali process, saturated brine enter to the chamber of anode cell, where chloride ions oxidized to chlorine gas and collect on the top of reaction chamber. Sodium ions pass through ion-selective membrane into cathode cell. water from the solution releasing Hydroxide ions and hydrogen gas. The sodium and Hydroxide ions become to caustic soda.
pH measurement is common use in order to maximize the facility efficiency.Particularly in the electrolysis reaction, the ion exchanger membranes are made by costly perfluorinated polymers, the pH changed in reaction has effect on the cell membrane lifespan.
After electrolysis process, spent brine flow into recirculation loop and back to brine treatment. Using Hydrochloric acid to remove any free chlorine from the brine.Control is typically at 2 to 4 pH prior to entering the dechlorination vacuum tower.Addition NaOH may add in the downstream ,thus, pH measurement is required before return to brine saturation tank.
Product Recommendation
- 2-wire pH/ORP analyzer
transmitter model: 96PH
sensor model: B65 (inline style)
B75 (insertion style) - 4-wire pH/ORP analyzer
transmitter model: 98PH
sensor model: B65 (inline style)
B75 (insertion style)







